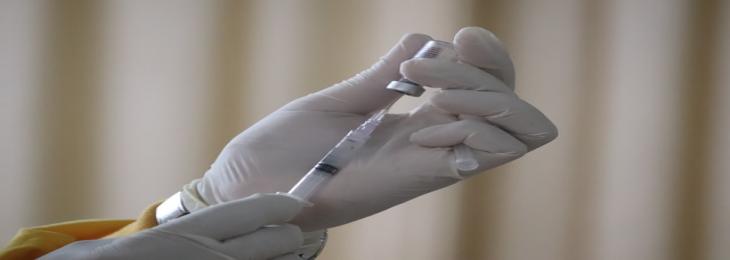
Heat Resistant COVID-19 Vaccine Ready for Human Trials
Aug, 2021 - By SMI
Share
For remote or resource-restricted sites with very hot climates lacking reliable temperature controlled chains, a thermo stable or harm vaccine is crucial.
Strong preclinical findings were achieved from a new COVID-19 heat tolerant vaccine. The vaccine can stay constant for up to 30 days at approximately 37ºC with scientists planning to start the human trials later this year. The requirement for the most vaccines to be maintained in cold conditions is a major challenge in distributing COVID-19 vaccines around the world efficiently. For instance, Pfizer and BioNTech vaccines for mRNA, which have a reputation for being ultra-cold, demand storage from -80ºC to -60ºC, which is why the delivery of theses vaccines to remote countries is difficult.
These temperature guidelines have recently been modified from Pfizer and normal freezer temperatures are found to be safe for at least two weeks. However, the distribution of the vaccine in large measure remains a challenge for cold storage supplies. In partnership with the Indian Institute of Science, the biotech startup Mynvax created the novel vaccine. Researcher’s discovered that the vaccine generates efficient, neutralizing SARS CoV-2 antibodies in the bodies of mouse and guinea pigs, as reported in the ACS Journal Infectious Diseases. This development of heat resistant vaccine was discovered to be remarkably stable at high climates. It works for 37ºC for a month and is reliable at saturation pressures of 100ºC for about 90 minutes. This successful preclinical findings paves the way for a human trial to begin later in 2021. Scientists expect that heat-resistant COVID-19 vaccines will help distribute vaccines to isolated regions with adequacy against latest variants of the COVID-19.
For remote or resource-restricted sites with very hot climates lacking reliable temperature controlled chains like Indo-Pacific regions, a thermo stable or harm vaccine is crucial, states Rob Grenfell, a scientist from CSIRO, a national science agency in Australia. The analysis suggested that every Mynvax formulas analyzed lead in antibodies lead in antibodies that were ready and willing to consistently neutralize the SARS-CoV-2 variants of the virus namely Alpha, Gamma, Beta and Delta.
Share
Stratagem Market Insights
533 Airport Boulevard, Suite 400, Burlingame, CA 94010, United States
Delivery Center
403, 4th Floor, Bremen Business Center
Aundh, Pune, Maharashtra 411007
India
